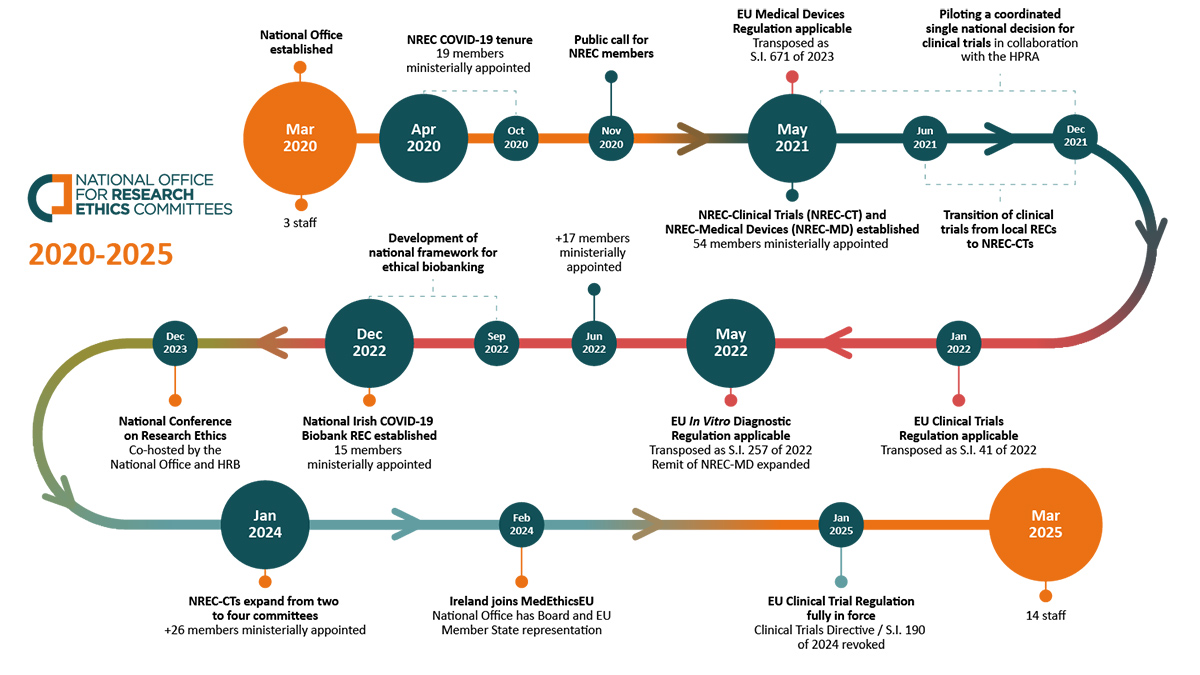
Since its establishment in 2020, the National Office for Research Ethics Committees, has been pivotal in reforming Ireland’s research ethics system for regulated research, becoming a crucial enabling pillar of the Irish health research landscape. Here, its Head of Office Dr Emily Vereker reflects on the significant growth and achievements of the National Office over the past 5 years.
 How it started
How it started
In March 2020, the National Office opened its doors in the Health Research Board, with an ambitious mandate to support National Research Ethics Committees (NREC) to deliver a single national ethics opinion for regulated studies – clinical trials, medical devices and diagnostic devices.
However, one week into its operations the National Office team of 3 rapidly pivoted, at the instruction of the then Minister for Health, to prioritise the immediate establishment of a NREC dedicated to providing expediated ethical oversight of all COVID-19 health research in Ireland. The COVID19 pandemic had already begun to shape the immediate work of the National Office and our future work to support areas of national strategic importance.
Over the course of 6 months, the NREC COVID-19 delivered 93 single national ethics opinions for COVID-19 research studies in Ireland. Ireland’s first national committee provided a proof of principle of the value of a national approach to research ethics in Ireland.
The Committees
Leveraging the experience from the NREC COVID-19, the National Office established statutory NRECs for clinical trials, medical devices and in vitro diagnostics in preparation for the EU Regulations coming into force in 2021. The mission and vision for the National Office team is to ensure the national research ethics system is sustainable, agile and has capacity within an ever-evolving regulatory research landscape in Ireland and across Europe. A restructure of the NREC-CTs, from a two-committee structure to a four-committee structure implemented by the National Office in collaboration with the NREC-CTs, brought sustainability and capacity to the system.
During this time, with COVID-19 remaining a key focus in health research, the National Office was mandated to establish a dedicated national research ethics committee to provide ethical oversight of the National Irish COVID-19 Biobank (NICB). The NICB-REC delivered a favourable national ethics opinion, underpinned by a robust ethical framework that may serve as a reference for any future ethics assessment of biobanking infrastructures in Ireland and beyond.
The Committee members
As the national system of ethics review is an integral infrastructure within the Irish health research ecosystem, it is critical that the NRECs are constituted with a diverse membership with skills, lived experience, interests and backgrounds reflective of Irish society. Our members are ministerially appointed and bring representative perspectives, diverse skills and competencies, subject matter expertise and lived experiences to their role.
The prevailing role of the members is to oversee best-practice ethical standards in regulated health research, ensuring the rights, safety, dignity, and well-being of research participants.
The national ethics review system would not succeed without the dedication of our committee members. Over the past 5 years we have seen the membership of the NRECs increase over time to build capacity and sustainability, growing from 54 members to over 100 members, with the majority committing to serve a second term on the NRECs.
The NRECs have collectively delivered a favourable single national ethics opinion for 505 new studies and 1,490 study modifications, over the course of 236 committee meetings.
The National Office has established and operationalised seven ministerially appointed NRECs for regulated research and areas of national strategic importance over the past 5 years and currently supports five NRECs with over 80 members in total.
The National Office team
Starting with just three staff, the National Office team has grown significantly into a vibrant team of 14 dedicated and high-performing professionals who strive to deliver an agile and trusted office in national public service.
The team are adept at navigating a complex and evolving regulatory environment with operational and technical expertise and has built a trusted and respected reputation for its expertise within the research community and national and international stakeholders.
The National Office team represent national interests at an EU level, driving harmonisation and implementation of EU Regulations through our participation in MedEthics EU, the COMBINE project, Accelerating Clinical Trials in the EU, and the Collaborate project. We’ve also developed a strong relationship with European colleagues through our membership with the European Network of Research Ethics Committees.
We built on our communication work, publishing blogs that are accessible to the public and developed specific guidance on challenging areas of ethics, for the benefit of the NRECs and Sponsors. In 2023 the National Conference for Research Ethics Co-hosted by the National Office and HRB brought together over 200 stakeholders such as health and social care researchers and practitioners, ethics committee members, patients and members of the public, to address some of the most burning ethical issues facing health research today.
Looking ahead
The growth and achievements of the National Office over the past 5 years have been supported by of our colleagues in the Health Research Board, the Department of Health, through our collaborative partnership with the Health Products Regulatory Authority and critically the NREC members that remain committed to supporting the national research ethics review system in Ireland. We’re grateful for their support, and that of our stakeholders.
As we look ahead, we will continue to support our members and advance the national research ethics system. We shall put further emphasis on importance of Patient and Public Involvement (PPI) in research ethics through engagement with our PPI members and national groups. Leveraging our collective knowledge and expertise to advocate for and guide on best-practice ethical health research, within Ireland and Europe will be a priority. The National Office will also work towards preparedness for emerging technologies and health threats through our engagement with national and European working groups.
The National Office eagerly anticipates the next five years, as we remain committed to enabling a progressive and ethically driven health research environment in Ireland.