NREC COVID-19
In 2020 as an emergency measure in response to the COVID-19 pandemic, the first NREC COVID-19 was established by ministerial appointment, to ensure a rapid national ethics opinion could be delivered for emergency COVID-19 research studies to facilitate the growth of evidence which would support population health.
As Ireland’s first NREC, the NREC COVID-19 provided proof of principle of the value of a ‘single national ethics opinion’ for Irish health research.
As the emergency circumstances under which the NREC COVID-19 and subsequent standing COVID-19 Subcommittee was set up to respond to, are no longer applicable, the COVID-19 Subcommittee is no longer in service as of 31 August 2024.
National Coordinated Response for Research Ethics
The NREC COVID-19 aligned its approach with the Health Research Consent Declaration Committee (HRCDC), and processes were ran in parallel to ensure robust, accelerated and coordinated review processes. The NREC COVID-19 maintained close contact with the Health Protection Regulatory Authority (HPRA), the national competent authority for clinical trials and medical devices in Ireland, with a view to ensuring consistency with regulatory review processes.
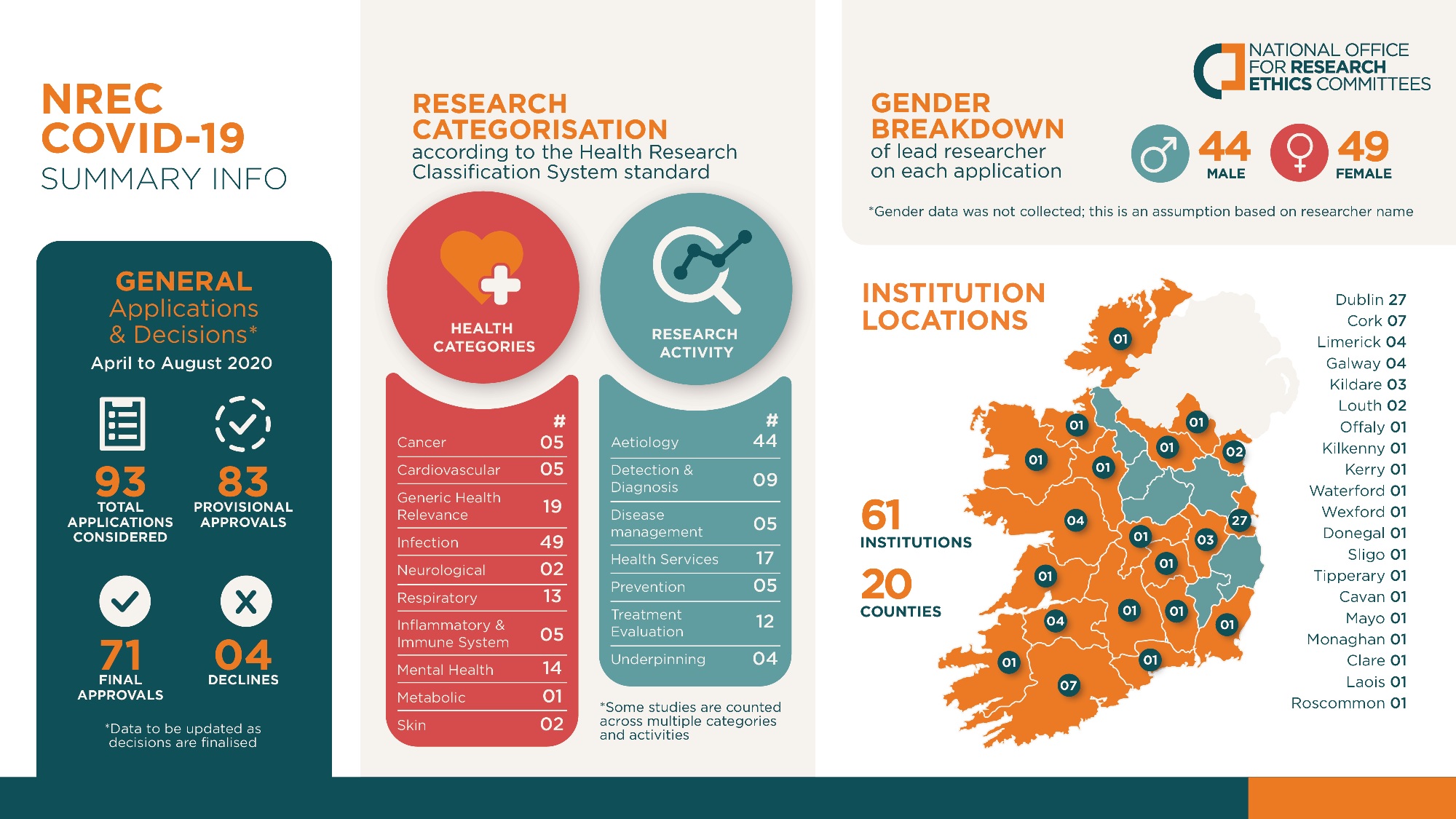
NREC COVID-19 Summary of Decisions
During its five-month tenure in 2020, on an expedited weekly basis, the NREC COVID-19 met frequently, mostly on an expedited weekly basis. The Committee returned decisions on a total of 93 applications for ethics approval; the vast majority of applicants received their outcomes within 24-48 hours of the committee meetings.
In summary, the NREC COVID-19 approved a total of 78 COVID-19 related-research studies. Following the end of this NREC’s tenure in August 2020, the standing COVID-19 Subcommittee has since reviewed a total of 49 amendments to approved studies over the last four years.
Demonstrating the value of the NREC COVID-19 over a relatively short period of time, is the breadth of its impact in terms of study type and location. Notably, approximately one quarter of research studies approved by the NREC COVID-19 involved an international collaboration.
Members of the NREC COVID-19 and COVID-19 Subcommittee contributed significantly to the establishment of the national research ethics review system in Ireland and were integral in supporting the emergency pandemic response at a national level.
The work of the National Office and NREC COVID-19 is published on HRB Open Research : Sheehy et al., 2020: Implementing a National Approach to Research Ethics Review during a Pandemic – the Irish Experience