As of Tuesday 31 January 2023, all applications for clinical trials in Ireland must be submitted through the one-stop online portal known as the Clinical Trials Information System (CTIS). In line with implementation of the EU Clinical Trial Regulation (CTR), this is now the mandatory system for the regulatory submission, authorisation and supervision of all clinical trials in the EU and European Economic Area (EEA).
The CTIS replaces the system previously in place under the Clinical Trial Directive (CTD), whereby applications were submitted directly to the National Research Ethics Committee for Clinical Trials (NREC-CT) or to the Health Products Regulatory Authority (HPRA). As a result, the National Office is no longer accepting submissions under the CTD for new trials.
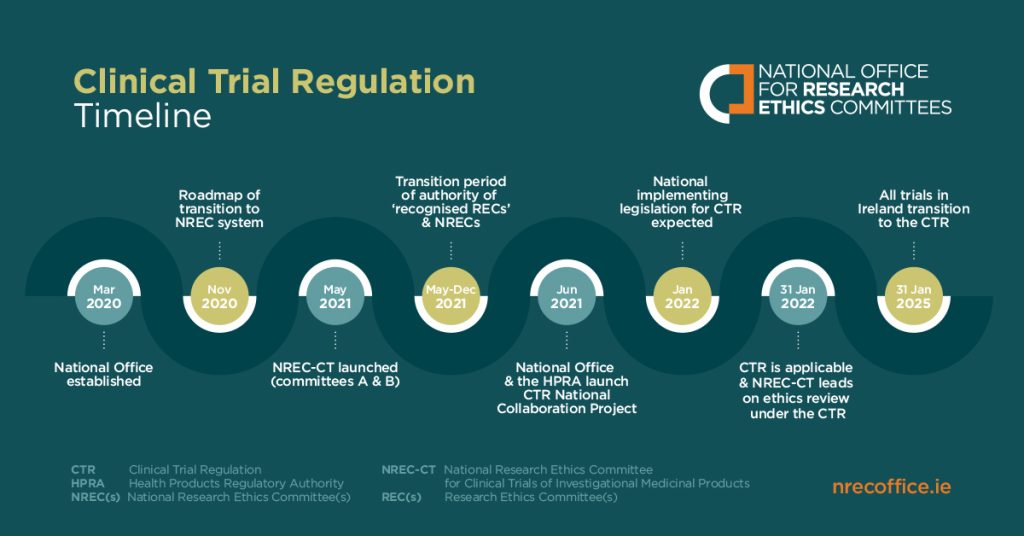
New/initial applications must be submitted under the Clinical Trials Regulation (CTR) using the Clinical Trials Information System (CTIS). Substantial modifications to trials authorised under the CTD are permitted until 30 January 2025. Please note however that all trials authorised under the CTD must either end or transition to the CTR by 30 January 2025.
The National Office website provides a number of resources to help applicants navigate this new system:
- An overview of the submission process under the CTR, including relevant document templates
- An introduction to the CTIS
- An overview of documentation under the Part II National Requirements
- An overview of the CTR and timeline for transition
For further queries related to the CTIS, please see the website of the European Medicines Agency (EMA). The EMA also offers a user support service for anyone working with the CTIS.

